Attitudes, Adoption Barriers and Projected Usage Timing for Pending Vaccine
Ongoing AMC Global research finds two-thirds of Americans willing to get an FDA-approved coronavirus vaccine, with different attitudes on preferred usage timing
Vaccine development for COVID-19 has been closely followed news since the pandemic began, with many seeing successful development as a "vital tool" to stopping this global health crisis. In this sixteenth wave of our ongoing study with OpinionRoute, we focused on Americans’ attitudes about usage of an FDA-approved coronavirus vaccine. While most respondents say they would agree to be vaccinated if a vaccine is approved this year, only some would want to receive the vaccine as soon as it is available, many would wait up to six months or more. For those who would not agree to be vaccinated, barriers included perceptions that there may not have been enough testing and concerns about safety.
You can see a full representation of the vaccine perception data here or click on the callout below.
Key findings for the week of August 24:
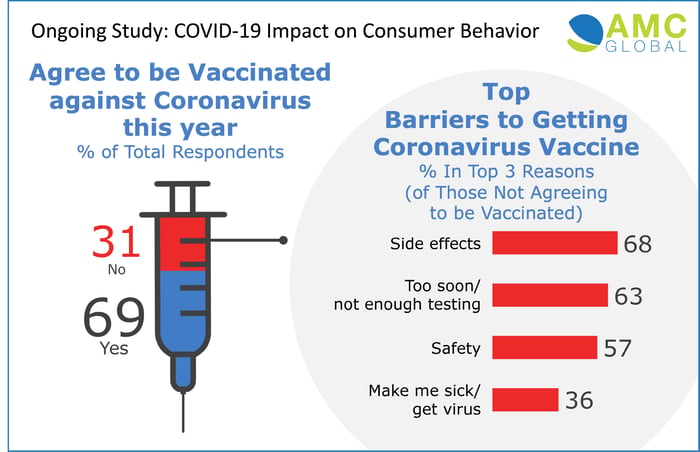
- Sixty-nine percent of respondents predict they will agree to be vaccinated against COVID-19 if an FDA-approved vaccine becomes available this year.
- Americans who would not agree to a vaccine approved this year report their top barriers are concerns about side effects (68%), insufficient testing (63%), and safety (57%).
- Timing on vaccination varies from ASAP (26%), after one month (18%), after two to three months (19%), after four to six months (8%), longer than six months (16%) to never (13%).
A complete graphical representation of the vaccine response data can be found here. New results and findings will be released on September 10.
It is essential to keep in tune with consumer attitudes and projected behaviors during this time. Contact us to learn more about effective and motivating product and brand positioning when trying to stay relevant in today’s world.